For a more comprehensive explanation, please refer to
(SI Appendix, section1).
Basic frame work of Vertex-Switcher using wireframe DNA oriagmi
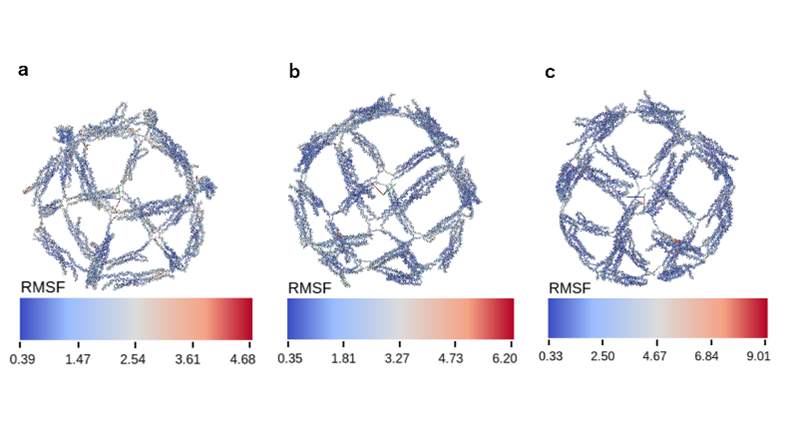
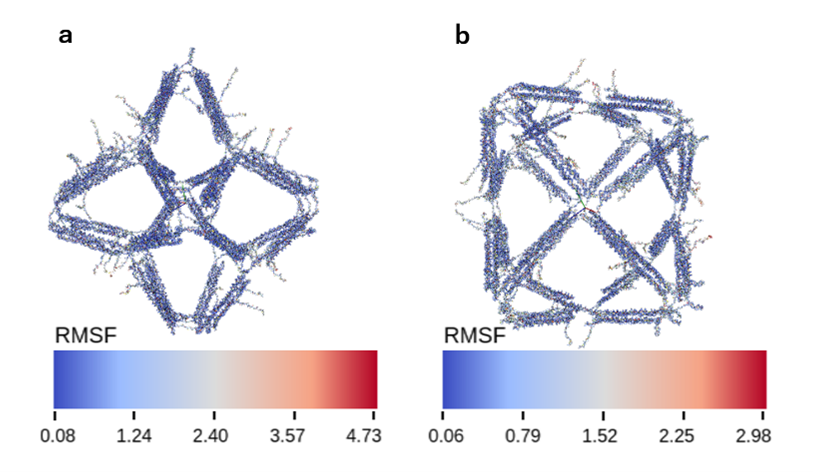
The default structure of Vertex-Switcher is a rhombic dodecahedron. This structure has six X-shaped vertices (Vertex Group X), each composed of four edges, and eight Y-shaped vertices (Vertex Group Y), each formed by three edges. By closing one of the Vertex Groups X or Y, the other vertex group retracts inside the structure, resulting in a structure with a different number of vertices. The addition of mechanism that enable such transformations allows us to consider the rhombic dodecahedron as an intermediate form that can transition between a hexahedral and an octahedral structure. We created this intermediate using wireframe DNA origami. Wireframe DNA origami creates a wireframe structure by hybridization of one long ssDNA (scaffold) to many short ssDNAs (staple). The length of one edge of this structure is about 25 nm, and the size of the intermediate structure is about 50 nm.
Fig.1 Dodecahedral Structure
Observation of this 3D model is possible through dragging.
4 helix Bundle for High rigidity edge
The goal of our project is to create DNA nanostructures that can be transformed into structures with different numbers of vertices by closing the vertices. However, if the structure consists of a single helix per edge, the edge may be deformed during the transformation. Therefore, to create DNA nanostructures that work as designed, the edges must be rigid structures. We ensured rigidity by constructing the edges of the structure with 4Helix Bundle. The 4 helix bundle is a structure of four DNA helices bound together by a crossover of staple strands and has higher stiffness than a single DNA helix.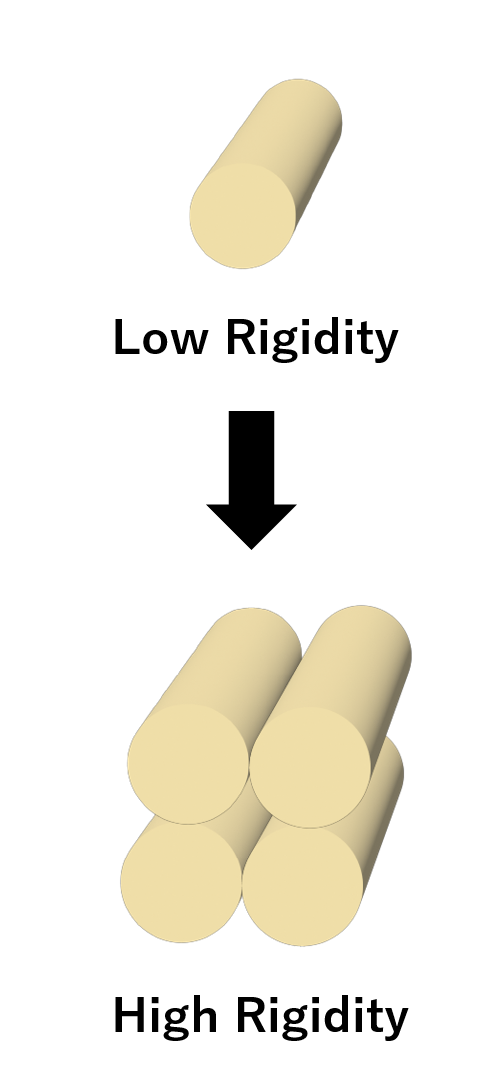

Fig.2 4 Helix Bundle
Fig.3 4 Helix Bundle Model
Observation of this 3D model is possible through dragging.
Strand displacement reaction for transformation the structure
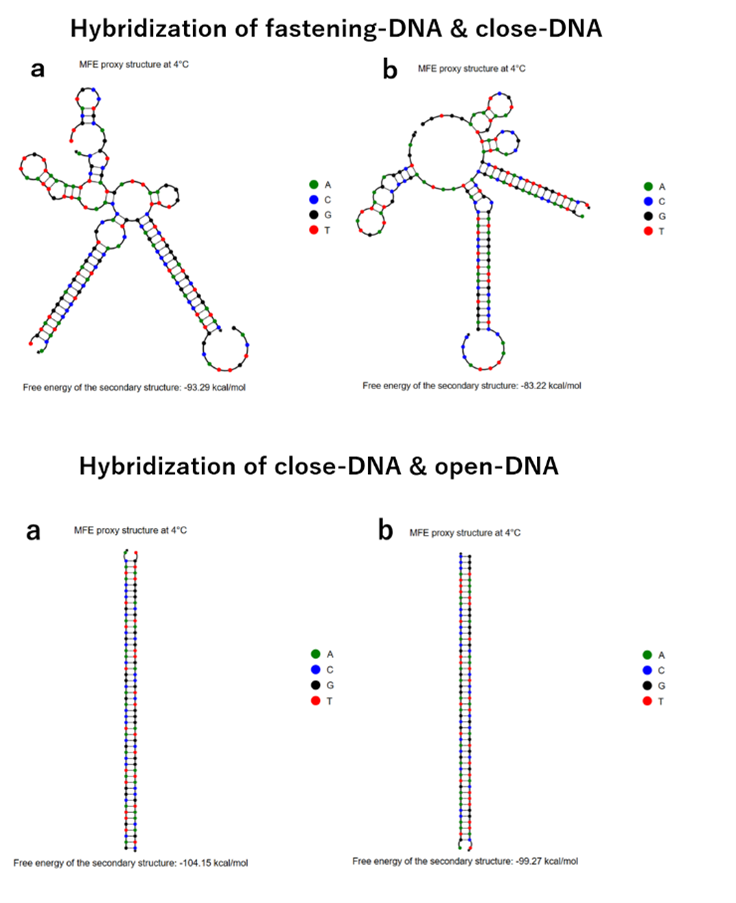
To enable the transformation from an intermediate form to other form, a specific angle-closing mechanism is necessary. We used the chain replacement mechanism for this purpose [2]. The mechanism consists of following components, including ssDNA (fastening-DNA) extending from each edge, ssDNA (close-DNA) that binds with fastening-DNA from opposing edges to close the angle, and signal DNA (open-DNA) that unites ssDNA strands. When close-DNA is added to the structure, open-DNA forms a double helix to bridge neighboring edges, pulling them closer and close the angle of vertex. By closing vertex group X, or Y in this way, vertices belonging to the other vertex group fall into the center of the structure to form a structure with a different number of vertices. When open-DNA is added to the structure with some of the vertices closed as described above, the open-DNA and close-DNA form a double strand, and the close-DNA is removed from the open-close mechanism ssDNA by strand displacement reaction, opening the angle of vertex. In this way, the DNA with its angle of all vertices opened returns to the intermediate form. Then, by adding close-DNA to that structure, it again becomes a structure with a different number of vertices.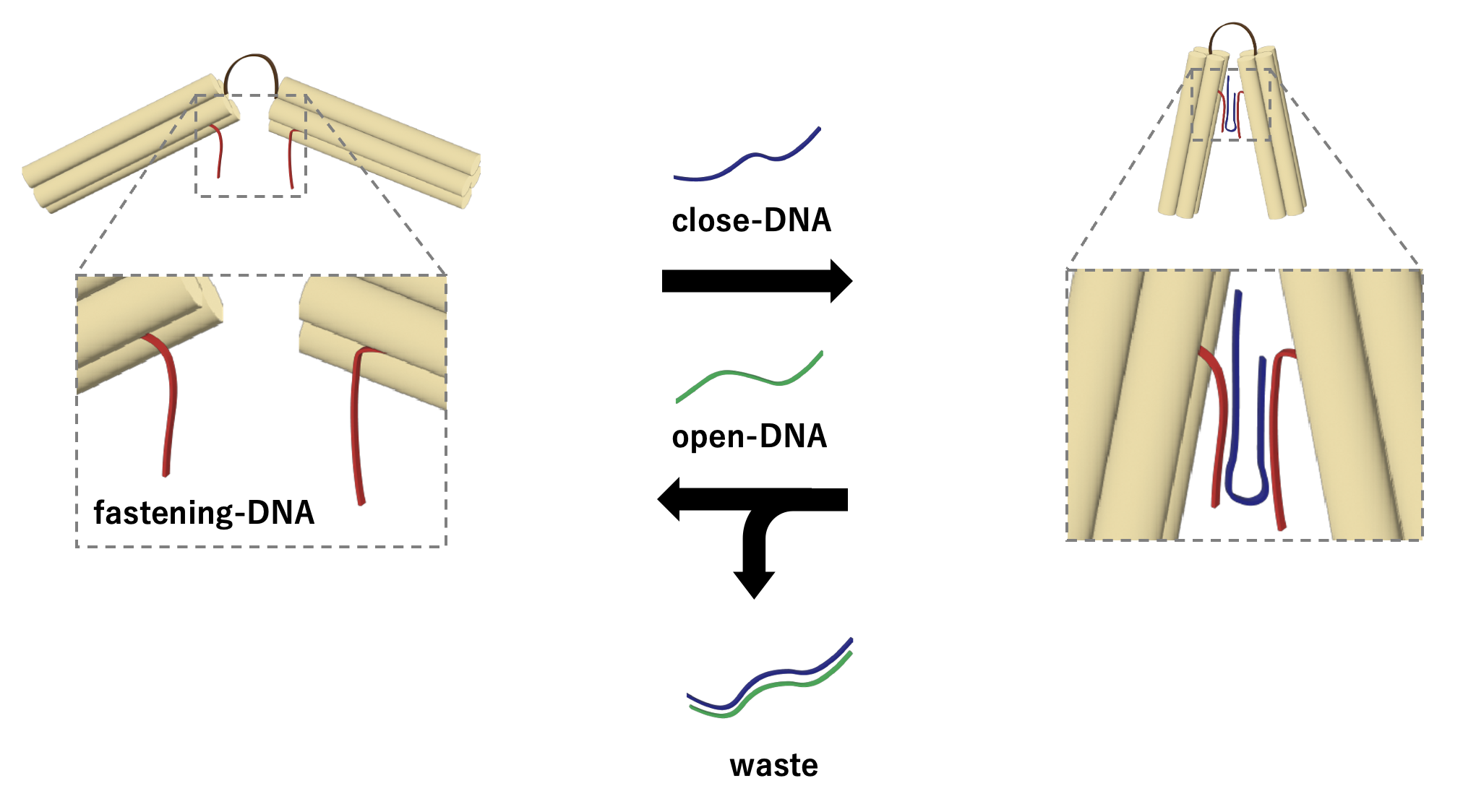
Fig.4 Strand displacement
Extension of hinges for improved mobility
In order to bring the edges closer together and close the angle of vertices, it is necessary to extend the length of the hinge portion connecting the edges to improve mobility. According to our calculations, the minimum necessary hinge length to constrict the angles formed by the edges to 30 degrees was approximately equal to 5 nucleotides of single-stranded DNA (ssDNA) [3]. Using this as a reference, we created following three patterns of hinge length to explore the optimal hinge length.: 3 nt (unaltered from the structure of the previous study), 6 nt (minimum length required for expected structural transformation), and 10 nt (sufficient length for structural transformation).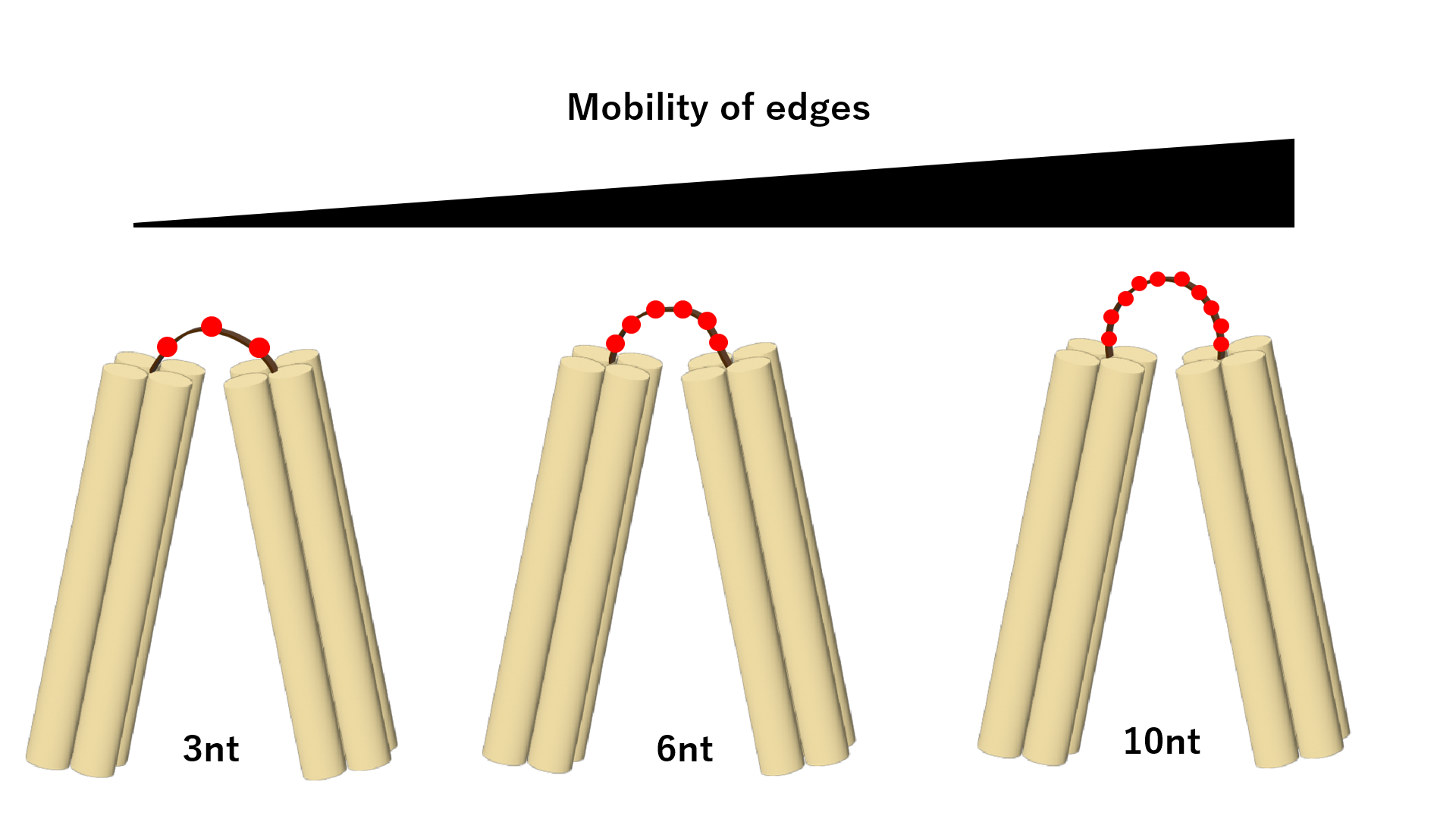
Fig.5 Hinge Elongation